In order to better implement the national normative requirements for drug clinical trials, adapt to new challenges, and improve the technical level of drug clinical trials, the quality of drug clinical trial management and the level of ethical review. The "GCP network conference" sponsored by Chengdu high-tech Medical Association was successfully held on September 24, 2021.

Professor Wu Zhengzhong of the professional committee of hospital pharmacy of Sichuan Hospital Association served as the chairman of the meeting and addressed the meeting; Professor Ning Hong of the pharmacy department of Mianyang Central Hospital was invited as the moderator, Professor He Lin of the pharmacy department (drug clinical trial center) of Sichuan Provincial People's Hospital and Professor Yang Jianhua of the pharmacy department of the First Affiliated Hospital of Xinjiang Medical University were invited as teaching experts to discuss "quality management and practice of clinical trial projects under the new GCP policy" and "requirements for the construction and filing of drug clinical trial institutions" Made a wonderful speech; Chen Yan, director of the drug / medical device clinical trial center office of Chengdu integrated traditional Chinese and Western medicine hospital, Ye Yongqin, director of clinical pharmacy of Chengdu Second People's Hospital, Shen Jing, director of Pharmacy Department of the Fifth Affiliated Hospital of Xinjiang Medical University, and Li Jing, director of Pharmacy Department of Banan District People's Hospital of Chongqing, had a heated discussion on key and difficult issues in the work process.

Speech by Professor Wu Zhengzhong of Hospital Pharmacy Committee of Sichuan Hospital Association
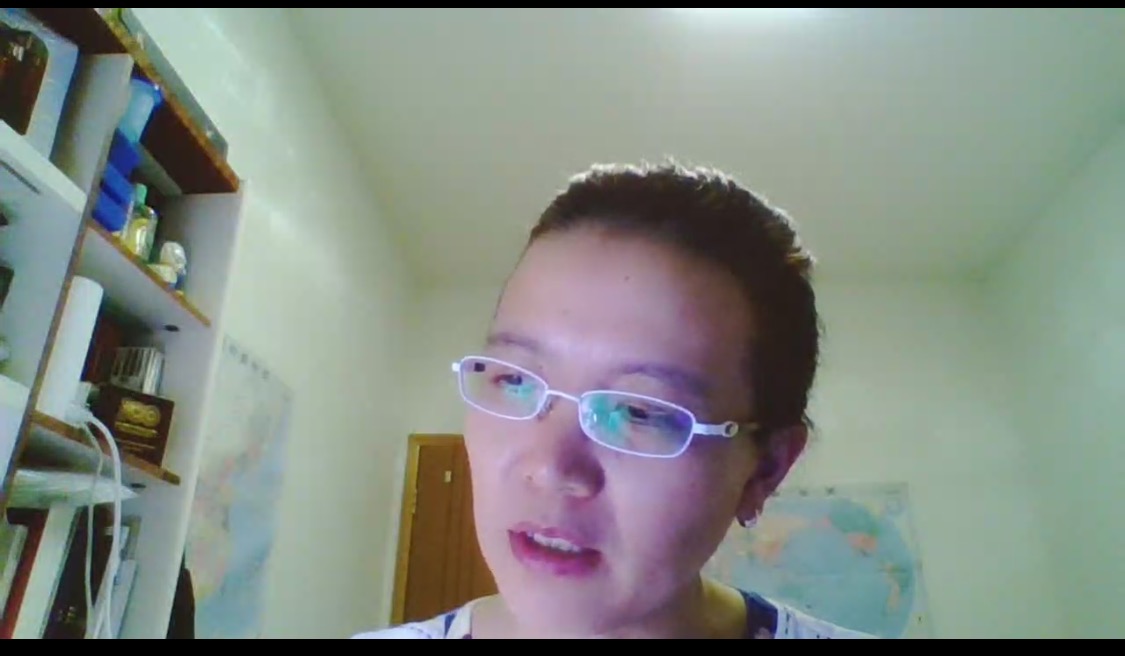
Ning Hong, director of Pharmacy Department of Mianyang Central Hospital
The experts invited to this meeting have rich practical experience and wonderful teaching contents, from which the students have benefited a lot. Through this meeting, the participants have further mastered the knowledge and skills of clinical trials and the quality management requirements of clinical trials of drugs and medical devices; At the same time, it also defines the responsibilities and obligations of researchers in the quality management of drug clinical trials, improves the quality of drug clinical trials, protects the rights and security of subjects, and ensures the standardized process and scientific and reliable results of clinical trials, so as to further promote the standardized research and high-quality development of drug clinical trials.

Yang Jianhua, director of the First Affiliated Hospital of Xinjiang Medical University, shared: quality management and practice of clinical trial projects under the new GCP policy

He Lin, director of Sichuan Provincial People's Hospital, shared: requirements for the construction and filing of drug clinical trial institutions
Wonderful moments for expert questions and discussions








