Entrusted by the Education, Culture and Health Bureau of Chengdu High-tech Zone, Chengdu High-tech Zone Medical Quality Control Management Service Center organized provincial and municipal review experts to conduct on-site review of the registration and filing of the second-level biosafety laboratory applied by Chengdu Fort Hays Medical Laboratory in the morning of May 7, 2022.

Chengdu High-tech Zone Medical quality Control Management Service Center has developed a technical review work plan according to the requirements of the second-level biosafety laboratory filing. Liu Hua from Sichuan Provincial People's Hospital and Chen Chuanrong from Chengdu Pangang Hospital were invited to form an expert review team for on-site inspection and acceptance. Zhu Lingqian, teacher of Health Department of Education, Culture and Health Bureau of Chengdu High-tech Zone, participated in the whole process of supervision, and the Medical Quality Control And Management Service Center of High-tech Zone participated in the organization and coordination.
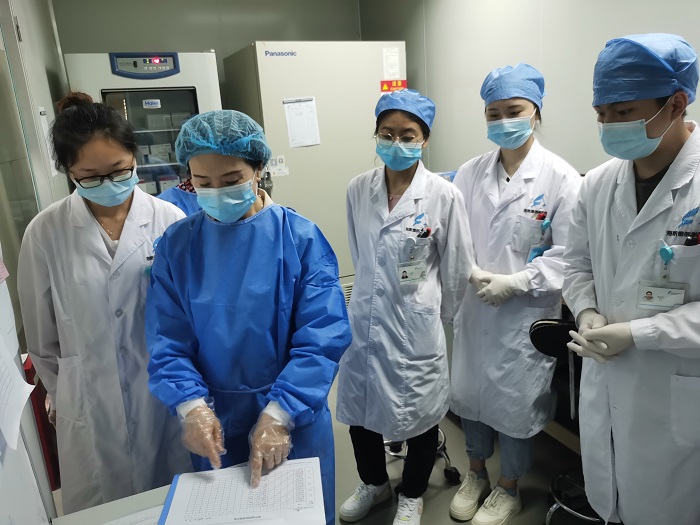
On the evaluation site, the two experts carried out a detailed inspection and acceptance of the division layout, personnel qualification, facilities and equipment, medical waste management, biological safety, system construction and other aspects of the laboratory of the unit, and gave valuable guidance, and put forward rectification requirements for the problems found in the evaluation process. This review has played a guiding and helping role in promoting the standardization construction and management of the laboratory.









